|
|
Abstract
Acute generalized exanthematous pustulosis (AGEP) is an uncommon acute
pustular eruption most often triggered by systemic drugs. We report a 68-years-old
female patient who developed AGEP after the intake of fenofibrate. To our
knowledge, this is the first report of this association in medical literature.
Therefore, fenofibrate should be added to the list of AGEP-causing agents.
Introduction
In 1980 Beylot et al. [1] introduced
the term pustuloses exanthemátiques aiguës généralisées (acute
generalized exanthematous pustulosis [AGEP] in English medical literature)
to rename the condition formerly termed exanthematic pustular psoriasis
by Baker and Ryan in 1968 [2], to describe
a subgroup of patients with pustular psoriasis who had a very acute pustular
eruption, drug intake and no history of psoriasis.
Fenofibrate is a lipid regulating agent of the fibrate class approved
as an adjunct to diet in the treatment of adult patients with primary hypercholesterolemia,
mixed dyslipidemia and hypertriglyceridemia [3,4,5].
Recent data also indicate its utility for optimizing reduction in the risk
of cardiovascular disease in patients with type 2 diabetes and metabolic
syndrome, as well as delaying the progression of diabetes-related microvascular
complications, when combined with a statin [4].
Cutaneous adverse reactions (CAR) attributed to fenofibrate are rare and
include acute hypersensitivity reactions such as severe skin rashes, urticaria,
photosensitivity reactions, contact dermatitis, Stevens-Johnson syndrome,
toxic epidermal necrolysis, pruritus, alopecia, herpes zoster, herpes simplex,
acne, sweating, nail disorder and skin ulcer [3,5,6].
AGEP had never been associated with fenofibrate therapy.
Case Report
A 68-year-old woman with atrial fibrillation, arterial hypertension and
diabetes mellitus was taking acenocoumarol (dose based on International
Normalized Ratio (INR) values), digoxin 0.2 mg/day, amlodipine 5 mg/day
and rosiglitazone/metformin 2 mg/1000 mg once/day for several years. Three
weeks before admission the patient was diagnosed with hypertrigliceridemia
and was prescribed fenofibrate 267 mg/day. After 7 days of treatment, she
developed an erythematous pustular eruption on inframammary folds, axillae,
inner surface of both arms and laterothoracic areas associated with subfebrile
temperatures. She attended her local doctor and was suspected to have scarlet
fever, being medicated with intramuscular penicillin. Because no clinical
improvement was achieved and the rash continued to spread, she returned
to her doctor 2 days later and was advised to cease penicillin, was medicated
with intravenous bolus of corticosteroid for suspicion of drug-induced cutaneous
reaction, and was referred for a dermatological opinion concerning diagnosis
and treatment.
On physical examination, we observed widespread edematous erythema over
the trunk, arms, buttocks, thighs and intertriginous areas, accompanied
by many small, nonfollicular, superficial pustules. On the neck, axillae
and inframammary folds confluence of pustules produced lakes of pus
(Fig. 1). The mucous membranes, palms, and soles were unaffected.
She was febrile (38.2
oC) and referred malaise and asthenia. She denied previous
therapy with fenofibrate and reported no adverse reaction to other drugs.
Personal or family history for psoriasis was negative.
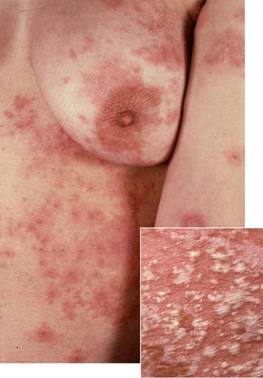
| Fig 1: Typical appearance of acute generalized
exanthematous pustulosis in our patient: disseminated
small non-follicular pustules on erythematous and edematous
skin. In some areas, confluence of pustules produced
lakes of pus (close-up). |
|
Routine blood tests revealed leukocytosis (12400/mm3; normal:
4000-11000/mm3) with neutrophilia (10400/mm3; normal:
2500-7500/mm3) and raised C-reactive protein (16.6 mg/L; normal
< 5.0 mg/L). The remaining blood panel, urinalysis and cultures obtained
from blood samples and pustular swabs were normal or negative.
The skin biopsy specimen demonstrated non-follicular intra- and subcorneal
pustules containing neutrophils, spongiosis, mild edema of the papillary
dermis and perivascular inflammatory infiltrate mainly composed of neutrophils
and eosinophils (Fig. 2). The histological findings were consistent
with the clinical diagnosis of AGEP.
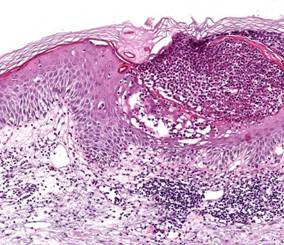
| Fig 2:
Histopathological
examination
showing subcorneal pustule with neutrophils and spongiosis
(H&E stain, 40x). |
|
Management involved withdrawal of fenofibrate, introduction of oral prednisolone
(30 mg/day with a 2-weeks tapering), bathing with antiseptic solution (potassium
permanganate) and emollients, which resulted in resolution of the exanthema
within 1 week, followed by thin desquamation. For lipid control, the patient
was prescribed simvastatin 20 mg/day.
About 2 months after the resolution of the dermatosis, skin patch tests
were performed with the Portuguese Contact Dermatitis Group standard series
and fenofibrate capsules (267 mg) at 1, 5 and 10% in petrolatum. The tests
were read at 48 and 96h and scored according to the International Contact
Dermatitis Research Group grading scale. Only positivity for potassium dichromate
(+) was detected. After a 6-month follow-up period there was no recurrence
of the skin lesions.
Discussion
The estimated incidence of AGEP is approximately 1 to 5 cases per million
per year [7,9].
More than 90% of cases of AGEP are drug induced, particularly by antibiotics
and mainly beta-lactams and macrolids [7,8].
Few cases are related to other causative factors such as viral infections,
mercurial antiseptics, topical agents, pneumococcal vaccine, herbal medications,
foods, ultraviolet light exposure and spider bite [7,8].
Bernard et al. [9] demonstrated a high frequency
of HLA-B51, -DR11 and -DQ3 in patients with AGEP. The interval between the
administration of the drug and the onset of the eruption is usually 2 or
3 days for antibiotics and longer (3-18 days) for drugs other than antibiotics.
Our patient started fenofibrate 7 days before the eruption.
The diagnostic criteria of AGEP, initially proposed by Roujeau et al.[8]
include: (i) numerous, small, non-follicular and sterile pustules arising
on a widespread edematous erythema, with burning and/or itching; (ii) fever
exceeding
38oC; (iii) histopathologic findings of subcorneal and,
sometimes intraepithelial spongiform pustules; (iv) blood neutrophil count
above 7000/mm3; and (v) acute course with spontaneous resolution
of the pustular eruption in less than 15 days, followed by a characteristic
postpustular pin-point desquamation. Recently, a validation score based
on morphologic and histologic criteria, and disease course was elaborated
by the EuroSCAR study group [7]. Our patient
had a classic drug-induced AGEP with typical morphology, course and histology,
fulfilling the diagnostic criteria established by Roujeau et al. [8].
Besides, according to the criteria of the EuroSCAR study group, fenofibrate-induced
AGEP was considered a definite diagnosis (12 points; range for definite
AGEP, 8-12).
Lymphocyte transformation tests and skin patch tests (SPT) can be useful
for diagnostic confirmation, and positive results suggest involvement of
T cells in AGEP [10]. The percentage of
positive SPT to the culprit drugs is frequently high (up to 80%) in patients
with AGEP, particularly for antibiotics [11].
However, negative tests do not exclude this diagnosis.
Early diagnosis of AGEP and the differentiation from other dermatoses,
such as generalized pustular psoriasis (von Zumbusch type), subcorneal pustular
dermatosis (Sneddon-Wilkinson disease), hypersensitivity syndrome with pustulation,
pustular vasculitis or even toxic epidermal necrolysis [7,8],
are important to avoid unnecessary and potentially dangerous drug therapy.
Additionally, as occurred in our patient, the combination of fever, leucocytosis
and pustules is often misdiagnosed as acute infectious disease leading to
unnecessary administration of antibiotics.
The withdrawal of the responsible drug is the main treatment for AGEP,
in combination with topical corticosteroids and antipyretics [1,7].
Because lipid lowering is effective for primary and secondary prevention
of cardiac events, one might expect an increase in the use of the various
lipid-lowering agents including fenofibrate and, as a result, the associated
CAR.
To our best knowledge there are no previous reports in the international
medical literature of AGEP induced by the ingestion of fenofibrate. Therefore,
this drug should be added to the list of potential causes of AGEP.
References
1.
Beylot C, Bioulac P, Doutre MS. Pustuloses exanthématiques aiguës généralisées,
à propos de 4 cas. Ann Dermatol Venereol. 1980; 107: 37- 48.
PMID: 6989310
2.
Baker H, Ryan TJ. Generalized pustular psoriasis: A clinical and epidemiological
study of 104 cases. Br J Dermatol. 1968; 80: 771- 793.
PMID: 4236712
3.
U.S. Food and Drug Administration. Drugs@FDA: Fenofibrate. Available at:
http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed
August 10, 2008.
4.
Zambon A, Cusi K. The role of fenofibrate in clinical practice. Diab Vasc
Dis Res. 2007; 4 Suppl 3: S15- 20.
PMID: 17935056
5.
Balfour JA, McTavish D, Heel RC. Fenofibrate: A review of its pharmacodynamic
and pharmacokinetic properties and therapeutic use in dyslipidaemia. Drugs.
1990; 40(2): 260- 290.
PMID: 2226216
6.
Roberts WC. Safety of fenofibrate: US and worldwide experience. Cardiology.
1989; 76(3): 169- 179.
PMID: 2673510
7.
Sidoroff A, Halevy S, Bavinck JNB, Vaillant L, Roujeau J-C. Acute generalized
exanthematous pustulosis (AGEP): a clinical reaction pattern. J Cutan Pathol.
2001; 28: 113- 119.
PMID: 11168761
8.
Roujeau JC, Bioulac-Sage P, Bourseau C et al. Acute generalized exanthematous
pustulosis: Analysis of 63 cases. Arch Dermatol. 1991; 127(9): 1333- 1338.
PMID: 1832534
9.
Bernard P, Lizeaux-Parneix V, Miossec V et al. HLA et predisposition génétique
dans les pustulosis exanthématiques (PEAG) et les exánthemes maculo-papuleux
(EMP). Ann Dermatol Venereol. 1995; 122: S38.
10.
Britschgi M, Pichler W. Acute generalized exanthematous pustulosis: a clue
to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr
Opin Allergy Clin Immunol. 2002; 2(4): 325- 331.
PMID: 12130947
11.
Wolkenstein P, Chosidow O, Flechet ML et al. Patch testing in severe cutaneous
adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal
necrolysis. Contact Dermatitis. 1996; 35: 234- 236.
PMID: 8957644© 2008 Egyptian Dermatology
Online Journal
|


